Autophagy is the procedure by which our cells recycle their parts. Most of the time it runs quietly in the background, however, if cells are stressed, autophagy becomes more important. Read on to learn about autophagy, its definition and how it functions, autophagy regulation, and what factors improve it.
What is Autophagy?
Autophagy (in the Greek for self-eating) is the controlled process by which a mobile degrades its dysfunctional or overseas components. The mobile can then recycle useful chemical elements for additional purposes.
This permits autophagy to regulate the equilibrium of protein composition within a cell, stop the buildup of toxic waste products, keep cellular organelle function, remove invading pathogens, and maintain cells during periods of low energy input because of fasting or starvation.
The scientific importance of understanding autophagy was highlighted when Yoshinori Ohsumi won the 2016 Nobel Prize in Physiology or Medicine for his discoveries of the mechanisms for autophagy.
Mechanism of Autophagy
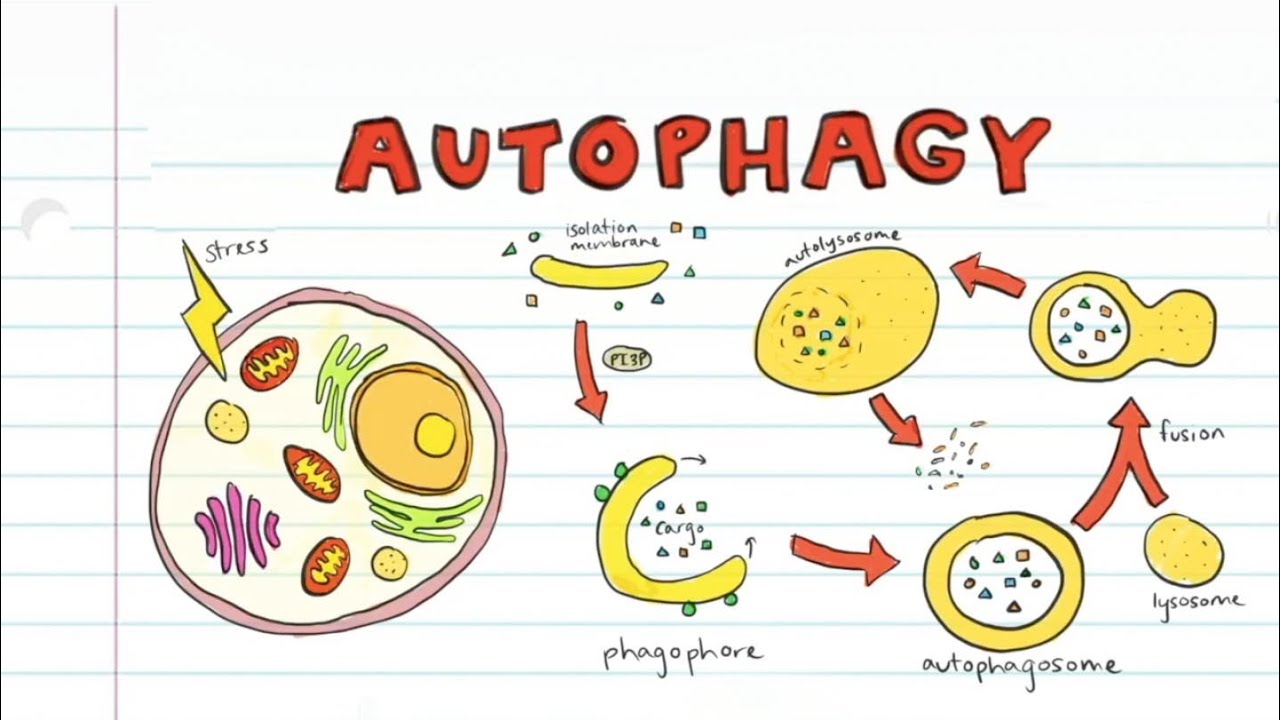
Essentially, autophagy is the creation of a ‘garbage bag’ (autophagosome) that accumulates cellular components and then takes them into the mobile’s ‘recycling center’ (lysosome) to be broken down into their parts that can then be recycled to new components.
Autophagy-related genes (ATG) are accountable for generating the structures that carry out autophagy. The VPS34 complex initiates the autophagosome, ATG9 contributes to its expansion, and the ATG12-ATG5–ATG16L1 complex recruits ATG8 proteins that complete formation and are included in concentrated capture.
Other genes are included with turning autophagy on and off. These genes can detect changes in the cell. mTOR responds to the level of nourishment in a cell and decreases autophagy (by disrupting ULK1 preventing the formation of the VPS34 complex) if there are plenty of nutrients available. AMPK monitors the energy levels within a cell (the amount of ATP) and activates autophagy when they’re low. HIF1A finds oxygen levels and turns on autophagy (targeting mitochondria) when they’re reduced (hypoxia).
Sirtuin genes (triggered by resveratrol) can inhibit mTOR which increases autophagy. Low levels of NAD+ are also reported to increase autophagy.
Research Limitations
Unfortunately, the mechanisms of autophagy are really difficult to study in humans, therefore the huge majority of the study dealt with this post was undertaken in animals and cells. This lack of clinical signs implies that not many conclusions can be drawn about autophagy and individual wellness.
The Objective of Autophagy
When things are running smoothly in a mobile, autophagy happens at a low level, helping to recycle drained mobile components. It is a kind of’maintenance’ mode.
Nevertheless, if things become worried in a cell (not enough nutrients or energy, dysfunctional components, or invasion by germs ), autophagy is turned up in order to help protect us. A ‘pressure response’ mode.
Aging & Lifespan
Activation of autophagy counteracts the age-associated accumulation of damaged cellular components and enhances the metabolic efficiency of cells.
Autophagy is a response to stress which helps cells to become more conservative and resilient with their energy.
In particular, autophagy could be activated to remove dysfunctional mitochondria (mitophagy) which produce a good deal of harmful reactive oxygen species (ROS) that hamper the cell.
These processes are reported to prolong the lifespan of several species.
Psychiatric Distress
The normal operation of autophagy offers protection against the development of psychiatric disorders. Disruptions to autophagic procedures have been associated with greater risk for several psychiatric ailments.
Post-mortem studies of the brains of people with depression and schizophrenia identified deficiencies in essential autophagy pathways.
Neurodegeneration
Many neurodegenerative disorders result from the accumulation of polyunsaturated fats in and around neurons, causing gradual brain cell death and subsequent lack of mental faculties.
Autophagy protects us from removing these proteins.
In Huntington’s disease, it removes the huntingtin (HTT) protein [15], in Alzheimer’s disease it removes amyloid ꞵ (created in the APP protein) [16], in Parkinson’s disease it removes ⍺-synuclein (SNCA), also in dementia it eliminates microtubule-associated protein tau (MAPT).
Infectious Diseases
Autophagy results in fighting infectious diseases in 3 ways;
- Immediate removal of germs from inside of cells (xenophagy)
- Removal of toxins created by diseases
- Modulation of the immune response to diseases
Infectious microbes (for example, Mycobacterium tuberculosis along with the Group A Streptococcus), viruses like HIV, and protozoans are removed through the processes of autophagy.
Infection
Autophagy can both increase and reduce inflammation reactions within the body. It increases inflammation by presenting signs of pathogen invasion and turning to the immune response.
Autophagy then reduces the inflammation brought about by an immune response by clearing the cell of antigens that are sparking the reaction. Additionally, autophagy additionally removes pro-immune reaction molecules produced by the cell in response to an invasion.
Muscle Performance
When working, we put stress on our cells. Energy use goes up, and cellular components can get worn out faster. Autophagy is then raised to be able to:
- Keep energy usage balance inside the cell
- Reduce the amount of external energy required (by more effectively recycling present energy molecules)
- Make sure degraded cellular components are removed before they begin to cause any problem
Cancer
Autophagy plays a part in preventing the onset of cancer and preventing the growth of early-stage cancers. Autophagy suppresses pro-cancer procedures like chronic inflammation, DNA damage response, and genome instability.
Mice genetically engineered to have impaired autophagy are reported to have increased rates of cancer.
Unfortunately, the protective roles of autophagy might also be hijacked by established tumors. As tumors grow the cells at the center become isolated in the blood supply and begin to experience nutrient and energy stress. Activation of autophagy can provide support to these cancerous cells. But, autophagy appears to be broadly beneficial before cancer cells can completely develop.
Factors That May Trigger Autophagy
Autophagy is a vital cellular process that is triggered when cells are under stress. As such, gentle or “hormetic” pressure can be useful if it disturbs autophagy without doing significant harm.
Alternately, some chemicals in food or supplements can trigger the mechanisms of autophagy.
Be aware that the relationships between autophagy and diet or lifestyle are extremely hard to demonstrate in human subjects. Therefore, the evidence for many such connections is confined to animal or cell studies, making it impossible to tell whether these variables increase autophagy in humans.
Use caution and talk with your doctor before starting any new supplement, exercise regimen, or lifestyle modification.
Mild stressors that may trigger autophagy include:
- Exercise
- Fasting or caloric restriction
Additional factors that may activate autophagy comprise:
- Diet/Foods
- Herbal remedies
- Supplements
- Active plant compounds
- Cancer treatment drugs
Lifestyle Factors
1) Aerobic Exercise
Aerobic exercise was demonstrated to induce autophagy in muscle tissues and the brain in animals, probably because of the prolonged stress related to physical exertion.
Does exercising make you feel fantastic and improve your health in other ways, but it also triggers autophagy to ensure your cells completely recover from the procedure.
2) Intermittent Fasting and Caloric Restriction
Irregular fasting and caloric restriction activated autophagy in animals.
A lack of nutrient influx triggers autophagy to increase the recycling of cellular components, enabling cells to continue to properly operate with less demand from outside resources.
This may be achieved either by opting to get a period without any food (fasting) or by reducing the amount of food you consume (caloric restriction). Irregular fasting was proven to induce profound neuronal autophagy and maybe a good method for combating neurological ailments.
3) Ketogenic Diet
The ketogenic diet reduces caloric consumption and increases fat intake, leading to a change of energy use from sugar into ketones. This shift imitates the process which happens during fasting and might thus cause the growth of autophagy.
According to animal research, ketogenic diets may promote autophagy from the brain, which many scientists have suggested is a vital mechanism for preventing esophageal diseases. However, this connection hasn’t yet been demonstrated in humans.
People who struggle with fasting or caloric restriction are often drawn to the ketogenic diet.
4) Sleep
Autophagy is activated during sleep. The circadian rhythm not only helps control your sleep cycle, but it’s also linked to autophagy. The inner biological clock affects the rhythm of autophagy.
In mice, sleep interruptions negatively influenced autophagy. When the animals’ sleep was interrupted, an interruption in autophagy protein transmission accompanied.
Foods
5) Coffee
Coffee has been proven to boost autophagy in mice.
A massive meta-analysis of over 340,000 people in the united kingdom found that just heavy drinkers (over six cups per day) experienced a higher rate of cardiovascular disease than nondrinkers, while moderate coffee drinking (one or two cups a day) was associated with decreased rates of CVD.
Although this study did not investigate autophagy especially, it demonstrates moderate ingestion of java is, at least not damaging to the center. Furthermore, impaired mechanisms and genes of autophagy are associated with CVD in animals.
No studies have investigated whether this speculative relationship between caffeine, autophagy, and CVD retains in people.
6) Green Tea/EGCG
As shown by a mouse analysis, active ingredients in green tea can activate autophagy. Specifically, polyphenols such as epigallocatechin-3-Gallate (EGCG) activated autophagy from the livers of mice awarded 3.2-gram EGCG per kg diet; the investigators settled on this dose as a rough equivalent to an individual drinking ten cups of green tea daily.
Folks would need to drink a lot of green tea to attain this kind of ingestion, and the link between EGCG and liver autophagy hasn’t been demonstrated in people.
Natural Supplements
7) Resveratrol
Resveratrol is a polyphenol found at low doses in berries, wine, peanuts, and soy.
Several studies have reported that an autophagy-inducing activity of resveratrol in cells.
In rats, caloric restriction and resveratrol collectively (but not either one alone) encouraged autophagy from the heart. However, this advantage has not been demonstrated in people, also resveratrol is notorious for its low bioavailability.
8) Curcumin
Curcumin has become the most active ingredient in garlic and contains many health benefits.
In a mouse study, curcumin activated autophagy and reversed some of the damage caused by osteoarthritis.
In cells, autophagy is reported to be triggered by curcumin (through the AMPK signaling pathway).
9) Vitamin D
Vitamin D is obtained through diet, supplements, and synthesized in the skin upon exposure to sunlight.
In mice and in cells that are isolated, vitamin D induced autophagy in pancreatic β‑cells, providing a potential avenue for use in diabetes. More studies are expected to ascertain whether the relationship between vitamin D, pancreatic autophagy diabetes holds in humans.
10) Omega-6 and 3 Polyunsaturated Fats
Supplements containing omega 6 and 3 polyunsaturated fats may raise autophagy.
In a study of rats with traumatic brain injury, omega-3 fatty acids encouraged autophagy and averted the death of neurons. In worms, omega-6 fatty acids improved the entire lifespan by triggering autophagy and slowing the processes of aging. Obviously, these outcomes have not been replicated in humans and must be taken with a grain of salt.
11) Nicotinamide
Nicotinamide is a part of the vitamin B family and a component for making the crucial energy molecule nicotinamide adenine dinucleotide (NAD).
In a mouse study, nicotinamide postponed the progression of cognitive decline and Alzheimer’s disease by raising autophagy in neurons.
Specifically, its ability to induce autophagic recycling of mitochondria could be beneficial to mobile health.
12) Lithium
Lithium is a chemical component that is reported to have many health benefits when used as a supplement.
Several studies have demonstrated that lithium is part of an autophagy-promoting signaling pathway in mammals. Lithium seems to induce autophagy by inhibiting inositol triphosphate (IP3), a protein that plays an important role in neuronal plasticity.
In creatures, lithium has been reported to improve the degradation of aggregate-prone proteins which cause Huntington’s disease, Alzheimer’s, and dementia. Some researchers attribute these effects to growth in autophagy.
13) Spermidine
Spermidine is a polyamine compound found in a wide range of foods.
Spermidine levels fall as people age, generating a correlation between spermidine and lots of the markers of aging. What’s more, diets rich in spermidine have been correlated with lower rates of cardiovascular disease and cancer. Spermidine has been found to activate autophagy in animals; some investigators have suggested that it could induce autophagy in humans and thus delay aging.
It has been reported to assist with cerebral declines and extend the lifespan of many organisms throughout the activation of autophagy.
Some of the foods with the highest reported levels of spermidine are dried noodles and one-year-old cheddar cheese.
14) Trehalose
Trehalose is a sugar that contributes to protecting the entire body.
It induces autophagy and has been reported to inhibit cytomegalovirus infection and also have neuroprotective properties. Much of the study on trehalose and autophagy was done in cells, but one study of maternal diabetes in animals found that trehalose claimed healthy levels of autophagy in the presence of excess sugar.
15) Other Compounds and Extracts
Many substances have been linked with increased autophagy in cell studies. The compounds in this list have not been shown to increase autophagy in animals, let alone humans; the evidence of the usefulness is therefore of very low quality and shouldn’t be applied as grounds to take them for any medical purpose. We include them because we think that it’s interesting to find out what compounds may be of interest to prospective research.
These chemicals and extracts comprise:
- Corynoxine, isolated from Uncaria rhynchophylla, promotes the usage of alpha-synuclein via the mTOR pathway.
- Cedrol, located in the essential oil of conifers, induces autophagy and cell death of lung carcinoma cells.
- Amla extract from Indian gooseberry (Emblica Officinalis) induces autophagy and inhibits ovarian cancer proliferation.
- Black hoof mushroom (Phellinus linteus) extract induces autophagy and inhibits breast cancer cell growth.
- Extract of European black nightshade (Solanum nigrum) induces autophagy and inhibits colorectal carcinoma cells.
- East Indian sandalwood oil induces autophagy and cell death in proliferating keratinocytes.
- Neferine from the Indian Lotus (Nelumbo nucifera) induces autophagy throughout the inhibition of both PI3K/Akt/mTOR pathway.
- Anacardic acids, found in the shell of the cashew nut (Anacardium occidentale), induce autophagy and inhibit lung carcinoma (A549) cells.
- Naringin, found in citrus fruits (especially grapefruit), causes autophagy-mediated growth inhibition of cancer cells.
- Astin B, from Aster tataricus, induces apoptosis and autophagy in liver cells
- Oridonin, purified by the herb Rabdosia rubescens, induces autophagy and cell death in prostate cancer cells.
Drugs
These medications have been found to induce autophagy in cells or animals; clinical trials (and so human signs ) are sorely lacking. We recommend strongly against taking one of these drugs without a physician’s prescription. If you’re already taking one, however, it can be interesting to note a possible impact on autophagy.
25) Medicines that Trigger Autophagy
Some drugs aim for the autophagic pathway. They can boost autophagy to assist clear bothersome targets (e.g. bacteria and dysfunctional proteins) from inside cells or they can use autophagy as a way of causing the death of cancer cells.
Always consult with a physician before taking any medication.
- Sirolimus, also known as rapamycin, causes autophagy by inhibiting mTOR.
- Fentanyl is a potent, synthetic opioid pain medication that induces autophagy through activation of their ROS/MAPK pathway.
- Carbamazepine, a medication used primarily for the treatment of epilepsy and neuropathic pain, induces autophagy and has been shown to act on multidrug-resistant Mycobacterium tuberculosis.
- Metformin, a diabetes drug, is an autophagic promoter that’s been trialed in Alzheimer’s therapy and reported to boost anxiety-like behaviors.
- Imatinib, a chemotherapy medication used to treat cancer, induces autophagy in chronic leukemia cells.
Bortezomib, an anticancer drug, induces autophagy in head and neck carcinoma cells.
Factors that Reduce Autophagy
The following substances are found to reduce autophagy in animal or cell studies, however, this isn’t necessarily (alone ) a motive to avoid them. This section should not be used as grounds to take or prevent a given supplement or medication. Talk to your doctor before starting any new supplement, and don’t under any circumstances stop treatment with medication before seeking your physician’s advice.
Supplements
Disclaimer
- Eugenol, a phenolic acid found in clove (Syzygium aromaticum), was identified from a display of 86 traditional Chinese medicines to get the strongest ability to decrease autophagy.
- Bafilomycin A1, a macrolide antibiotic derived from Streptomyces griseus, has been reported to inhibit autophagy.
- Elaiophylin was identified from a display of North China Pharmaceutical Group Corporation’s pure compound library of the microbial sources to have the best ability to decrease autophagy.
- Oblongifolin C, a phenolic acid in the Asian shrub Garcinia yunnanensis, inhibits autophagy and has been described as having anticancer properties.
- Matrine, an alkaloid found in plants from the Sophora genus, is reported to be an autophagy inhibitor (via modulation of this lysosomal procedure).
Medicines
- Wortmannin
- 3-methyladenine
- Spautin-1
- Clomipramine
- Lucanthone
- Chloroquine
The Genetics of Autophagy
Autophagy is closely controlled by turning off and on autophagy-related genes (Atg). Individual genetic differences may strongly influence this procedure.
Genes Involved with the Mechanism of Autophagy
Autophagy begins with the inception of a phagophore from membranes improved with phosphatidylinositol 3-phosphate (PI3P).
This enrichment is aided by a group of proteins known as the’VPS34 complicated’ (VPS34, the regulatory subunit p150, BECLIN 1, and ATG14).
Expansion of this membrane area is eased by ATG9 which cyclically introduces more lipids into the bi-lipid membrane.
The phagophore structure takes shape via the activity of these proteins CapZ and WHAMM.
During maturation the ATG12-ATG5-ATG16L1 complex recruits ATG8 proteins (particularly cleaved LC3B and GABARAP). These help with morphing to the ‘tote’ shape of the autophagosome.
One of the ATG8 family members, LC3A, B, and C are largely concerned in autophagosome formation while GABARAP, GABARAL1, and GATE-16 are involved later in the maturation measure.
While autophagy is usually viewed as a random procedure, engulfing mobile components, it seems to also have the ability to target certain elements for degradation.
LC3 is reported to possess another function wherein it incorporates cellular components that are exposing a particular arrangement (the LIR motif).
The targeting of old parts of the endoplasmic reticulum and nuclear membrane is facilitated by the FAM134 reticulon protein family.
NDP52 and OPTN are the main molecules involved in targeting mitochondria.
Components (proteins and Illness ) tagged for degradation (with ubiquitin) are targeted by p62.
The freight of an autophagosome is broken down and recycled from the contents of a lysosome. Attachment and fusing with both are eased by an interaction between ATG14, STX17, and SNAP-29.
Genes Involved in Autophagy Activation
The majority of autophagy is controlled by the mammalian target of rapamycin (mTOR). When nutrients are limited, mTOR is inactivated, which subsequently induces autophagy.
MTOR suppresses the ULK complex (ULK1/2, ATG13, FIP200) which ends up on the VPS34 complex and begins phagophore ‘budding’.
AMPK is a key sensor of intracellular energy under conditions of starvation or ecological stress. It can turn on autophagy by acting on the ULK complex.
AMPK also can inactivate mTOR via the tuberous sclerosis complex (TSC) which prevents mTOR from obstructing autophagy.
You will find other mechanisms for turning on and off autophagy which doesn’t involve mTOR/AMPK. The inhibition of inositol monophosphatase and disruptions to the endoplasmic reticulum (causing the production of faulty proteins) can both turn on autophagy.